Covid vaccine: Moderna seeks approval in US and Europe
Regulators will look at trial data for the mRNA vaccine and decide if it is safe and effective enough to recommend for roll out.
Clinical studies show the jab is more than 94% effective at protecting people from becoming ill with Covid-19.
Pfizer, which has a similar jab, has already filed for the same US approval.
UK regulators are also reviewing data on the Pfizer vaccine, as well as another type of Covid vaccine from
AstraZenca and Oxford University for emergency approval.Moderna says it hopes to gain UK approval soon, now that it has trial data from 30,000 volunteers - including high risk groups like the elderly - that suggests it works.
In those studies, 15,000 people received the real vaccine while the other participants got placebo injections. No serious side effects were reported.
During the studies, 185 people in the placebo group fell ill with Covid-19, and some severely so.
In comparison, there were 11 cases in the vaccine group and none were severe.
Full trial data has not been released, but will be published in a peer-reviewed journal in due course.
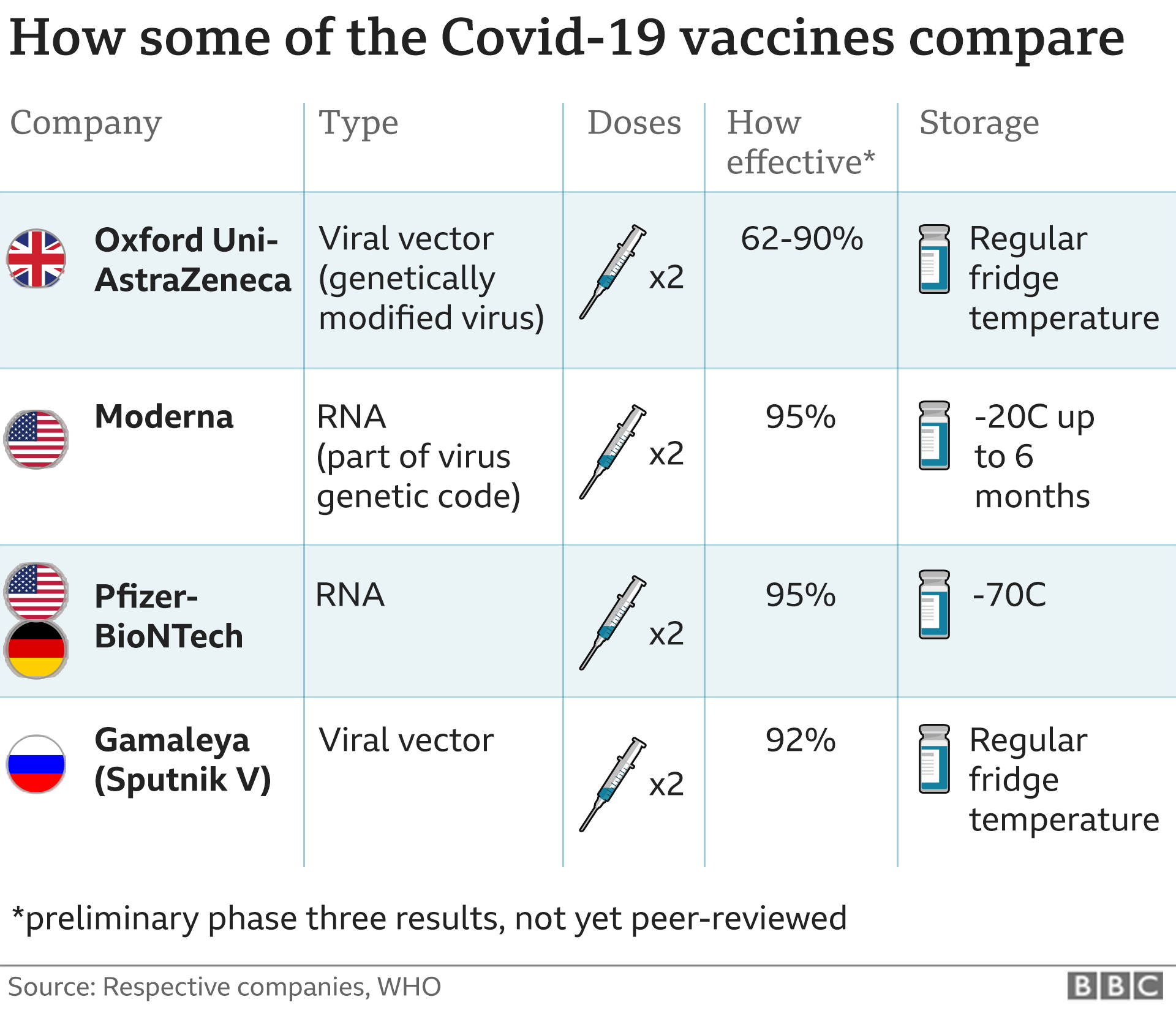
The three front-runner vaccines have different pros and cons.
The AstraZeneca jab is cheaper - around £3 ($4) for a dose, compared to around £15 ($20) for the Pfizer vaccine and £25 ($33) for Moderna's.
And it is potentially easier to distribute because it does not need to be stored under ultra-low temperatures.
But its efficacy in trials - between 62% and 90% - is a bit lower than the Pfizer and Moderna vaccines.
The UK has already pre-ordered doses of all three vaccines:
- 7m of the Moderna jab
- 40m of the Pfizer/BioNTech one
- 100m of the AstraZeneca Oxford vaccine
Dr Alexander Edwards, associate professor in biomedical technology at the University of Reading, said: "This is great news indeed - the more trial data that we have, the greater confidence we have that vaccines can be used to blunt the human cost of Covid-19.
"As the numbers of cases reported grows, confidence grows that this amazing protection will be maintained in a product that can be rolled out to protect the public."

December 01, 2020 at 01:00AM
https://www.bbc.co.uk/news/health-55129336
Labels: BBC News

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home