Covid: AstraZeneca contract must be published, says European Commission chief

The head of the European Commission, Ursula von der Leyen, has called for the EU's vaccine contract with drug-maker AstraZeneca to be published, in a growing row over reduced supplies.
The contract signed in August contained "binding orders", she told German radio, and she demanded "plausible explanations" for the hold-ups.
UK-Swedish AstraZeneca is blaming production delays at two plants.
Its vaccine is expected to be approved by the EU medicines regulator later.
The August deal was for 300 million doses for the European Union to be delivered after regulatory approval, with an option for 100 million more.
But EU sources say they now expect to get only about a quarter of the 100 million vaccines they were expecting to receive by March, a shortfall of about 75 million jabs.
AstraZeneca says the production problems are at its plants in the Netherlands and Belgium.
The EU wants the contract published to back up its argument that AstraZeneca is reneging on its commitments.
The company's chief executive, Pascal Soriot, has said that the contract stipulated that the company would make its "best effort" to meet the EU demand and did not compel the company to stick to a specific timetable - an assertion disputed by the EU.
The EU is under pressure after criticism that the pace of vaccine distribution in several member countries has been too slow.
Supplies of another vaccine, produced by Pfizer-BioNTech, have also dropped due to production issues.
Warning of a 'vaccine war'
"There are binding orders and the contract is crystal clear," Mrs von der Leyen said in Friday morning's radio interview.
"'Best effort' was valid while it was still unclear whether they could develop a vaccine. That time is behind us. The vaccine is there.
"AstraZeneca has also explicitly assured us in this contract that no other obligations would prevent the contract from being fulfilled," she said.

The company is producing the jab at its UK plants too and there have been no reported problems with its contract with the UK authorities.
EU officials say AstraZeneca has been asked to send some doses manufactured in the UK to the continent to make up the shortfall, but the company said on Wednesday that its contract for UK supplies prevented this.
UK Cabinet Office Minister Michael Gove said on Wednesday that UK supplies "won't be interrupted".
But Mrs von der Leyen insisted the EU's contract with AstraZeneca listed two UK plants as production sites for vaccine destined for the EU.
Calling for the document to be published, she said: "We are speaking with the company about which parts need to be redacted. But we want to achieve transparency."
The EU is likely to unveil special powers later to help ensure its supply of vaccines, including a possible limit on the export of vaccines produced in the bloc.
There is speculation that these powers could also see companies being forced to hand over production to other firms inside the EU and share intellectual property.
However, the European Council is stressing the need for negotiations in order to reach a solution before enforcement becomes necessary.
Meanwhile, EU Justice Commissioner Didier Reynders has warned of a "vaccine war".
Speaking on Belgian radio, he said: "The EU commission has pushed to co-ordinate the vaccines contracts on behalf of the 27 precisely to avoid a vaccines war between EU countries, but maybe the UK wants to start a vaccine war?
"Solidarity is an important principle of the EU. With Brexit, it's clear that the UK doesn't want to show solidarity with anyone."
Vaccine approval awaited
The European Medicines Agency is expected to grant approval to the AstraZeneca vaccine later, with an announcement due at 14:00 GMT.
The regulator's decision is keenly awaited, in part to see whether or not it will approve the jab for use in over-65s.
Germany's vaccine commission decided against doing so on Tuesday, saying there was not enough data from that age group.
AstraZeneca and the UK regulators, the MHRA, have said they are confident the jab provides protection in all age groups.
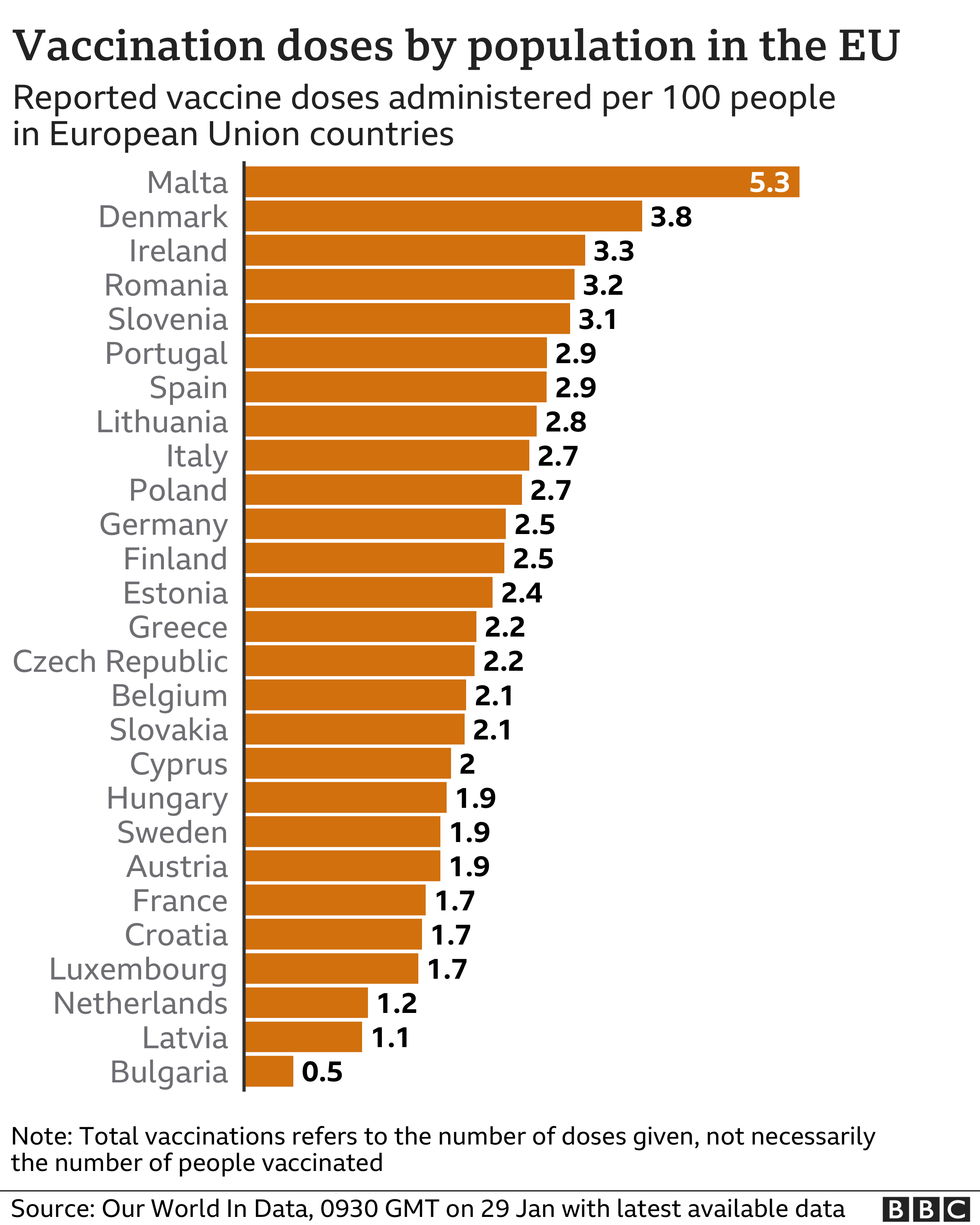
Supplies run low in Europe
Vaccinations in parts of Europe are already being held up and in some cases halted because of a cut in deliveries of the Pfizer-Biontech vaccine:
- In Spain, Madrid and the northern Cantabria region have halted first vaccinations to focus on second doses for at least two weeks.
- Regional health authorities in France are delaying vaccination appointments. More than 1.1 million people have received a jab so far
- Vienna's city councillor for health says delivery problems are leading to delays in vaccinations by up to two weeks. "We are really operating in a dramatic form of shortage economy," said Peter Hacker
- The Dutch government was the last in the EU to start a vaccination programme and by the end of January the Netherlands will have had no more than 757,000 doses, mainly from Pfizer. It initially based its strategy on the assumption the AstraZeneca vaccine would be available first
- The head of Croatia's public health institute says Pfizer has reduced the number of doses for the next three shipments and all the doses in Croatia are now being kept for second shots

January 29, 2021 at 09:45PM
https://www.bbc.co.uk/news/world-europe-55852698
Labels: BBC News

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home