Covid-19: US agencies call for pause in Johnson & Johnson vaccine
US health authorities are calling for a pause in the use of the Johnson & Johnson Covid-19 vaccine, after reports of extremely rare blood clotting cases.
The Food and Drug Administration (FDA) said six cases in 6.8 million doses had been reported and it was acting "out of an abundance of caution".
Johnson & Johnson said it was also delaying its vaccine rollout in Europe.
The US move follows similar rare cases in the AstraZeneca vaccine, which has prompted some curbs in its use.
The US has by far the most confirmed cases of Covid-19 - more than 31 million - with more than 562,000 deaths, another world high.
The picture for the virus in the US is complicated, though, with some areas in the north seeing surges in infections, the south less, and with the figures not always reflecting inoculation numbers.
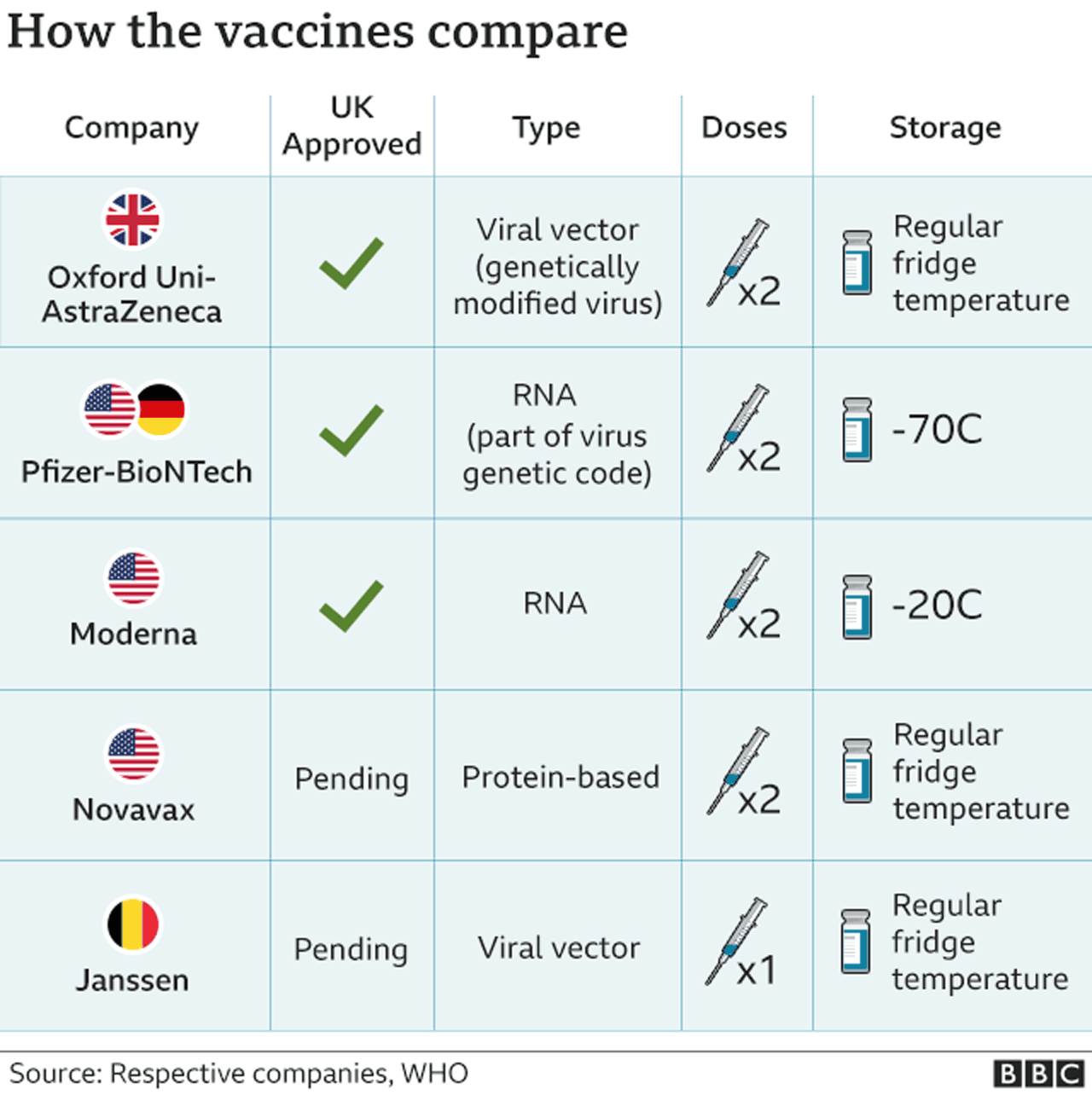
The Johnson & Johnson jab was approved in the US on 27 February and its use has been more limited so far than that of the Pfizer-BioNTech and Moderna doses.
Nevertheless, the government had hoped for hundreds of thousands of vaccinations of the jab every week as it is single-shot and its storage at common refrigerator temperatures makes it easier to distribute.
It is also known as the Janssen vaccine, named after the Belgian company that makes it.
South Africa became the first country in the world to administer the jab, and nearly 300,000 health workers have received it since mid-February. Health authorities there have not yet decided whether the roll out will continue.
The vaccine is yet to be approved in the UK, although 30 million doses are on pre-order. The Department of Health said the rollout delay would not affect vaccine supplies in the UK, or derail the aim to offer a jab to all adults by the end of July.
What is the recommendation?
In a joint statement, the FDA and the Centers for Disease Control and Prevention (CDC) said they were "reviewing data involving six reported US cases of a rare and severe type of blood clot in individuals after receiving the J&J vaccine".
It said the clotting was called cerebral venous sinus thrombosis (CVST).
The statement said that this type of blood clot needed a different treatment than usual.
The common treatment - an anticoagulant drug called heparin - "may be dangerous", it said.
Pending a further review, the FDA and CDC recommended "a pause in the use of this vaccine out of an abundance of caution".
This was to "ensure that the health care provider community is aware of the potential for these adverse events".
All six cases were in women aged between 18 and 48, with symptoms appearing six to 13 days after vaccination.
One patient died from blood clotting complications, while another is in a critical condition, the FDA's Peter Marks confirmed.
The joint statement said that "people who have received the J&J vaccine who develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider".
The federal government is now likely to pause the use of the vaccine in all federally run vaccination sites, and to expect state sites to do the same.
Johnson & Johnson statement
Johnson & Johnson, a US health care company, issued a statement saying that safety was its "number one priority" and that it shared "all adverse event reports" with the health authorities.
It added: "We are aware that thromboembolic events including those with thrombocytopenia have been reported with Covid-19 vaccines. At present, no clear causal relationship has been established between these rare events and the Janssen (J&J) Covid-19 vaccine."
It also said that it had been reviewing cases with European health authorities.
"We have made the decision to proactively delay the rollout of our vaccine in Europe," it said.

Cautious approach for the incredibly rare
By Rachel Schraer, BBC health reporter
The US health protection agency has identified a very small number of the same rare form of blood clots seen in people given the AstraZeneca jab.
Governments around the world have cautiously begun to link these rare blood clotting incidents to the vaccine because of their unusual presentation - though this link hasn't been definitively proven.
People suffering them had very low platelet counts - blood cells that normally help repair damage in the body.
The Johnson & Johnson vaccine works in a very similar way to the AstraZeneca one, so in some ways it's not surprising they may cause similar side effects. And they appear to be comparably rare.
The numbers we're talking about are so low that it's difficult to say confidently what the risk of fatal blood clots is, but for the AstraZeneca jab it has been estimated at one-in-a-million. There have been six cases out of 6.8 million doses of the Johnson & Johnson jab.
In contrast, Covid kills one in 1,000 infected in their 40s among those who develop symptoms (and this risk is much higher among older people).
Once you get into the youngest age groups, who are less likely to die from Covid, that risk calculus shifts, particularly when there aren't too many infections in circulation.

AstraZeneca vaccinations
The Oxford-AstraZeneca vaccine, which has been given safely to tens of millions of people, has also seen some extremely rare blood clotting cases.
The reports led some nations to suspend its use but most have now resumed, although in a number of cases with a recommended minimum age, for example 60 and over in Germany.
In the UK, authorities advised that those under 30 should be offered an alternative.
The delay in rolling out Johnson & Johnson in Europe, along with production and supply problems for AstraZeneca, could mean worsening problems for the European vaccination drive.

April 14, 2021 at 04:12AM
https://www.bbc.co.uk/news/world-us-canada-56733715
Labels: BBC News

0 Comments:
Post a Comment
Subscribe to Post Comments [Atom]
<< Home